What is ORP in water?
ORP stands for Oxidation-Reduction Potential. It is a measure of how well a solution can gain or lose electrons. This helps us understand if the solution tends to oxidize or reduce substances.
In liquid systems, ORP is the voltage difference between a redox electrode and a reference electrode. This measurement gives a clear picture of the redox state of the solution.
A positive ORP value shows an oxidizing environment. Higher values mean stronger oxidizing properties. Water with substances like chlorine, ozone, or a lot of dissolved oxygen often has a high positive ORP value. This makes it better at capturing electrons from other materials.
A negative ORP value means a reducing environment. Lower values mean a stronger ability to give away electrons. For example, in low-oxygen or anaerobic conditions, water often shows a negative ORP value. This occurs when reducing agents such as hydrogen sulfide, ferrous ions, or a lot of organic matter are present.
The ORP level in water varies based on how it is used. Drinking water usually has an ORP between +200mV and +600mV. Swimming pools or clean water can have levels higher than +650mV. This level offers strong benefits against germs.
Aquaculture environments usually have ORP values between +250mV and +400mV. Sewage systems often have lower ORP values. Sometimes, these values can drop below 0mV or even down to -200mV in extreme cases.
How is ORP measured?
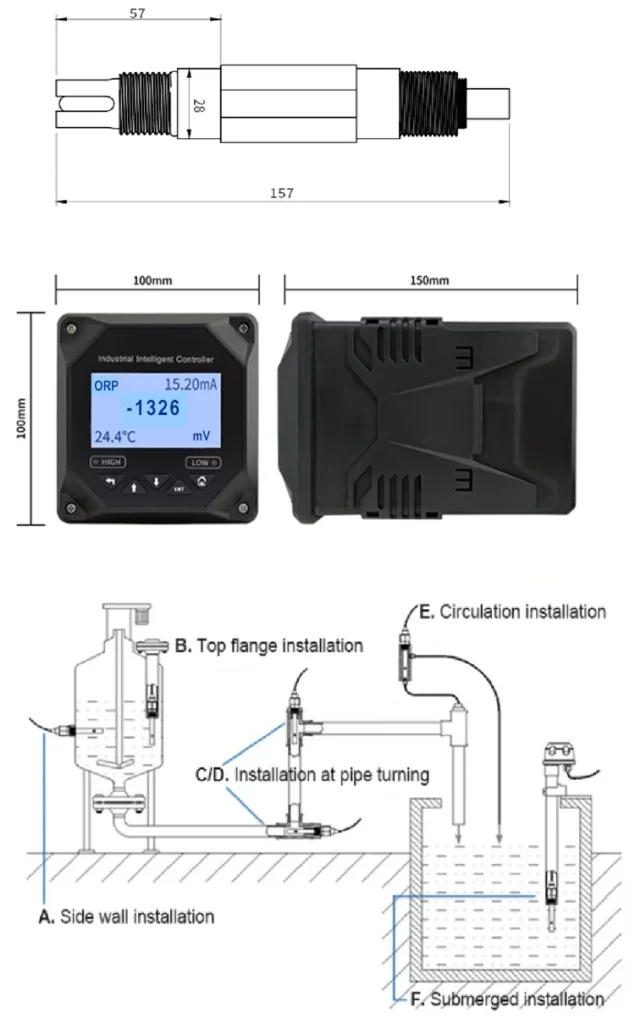
ORP is measured in millivolts (mV) with special ORP sensors or probes. These devices are placed in the liquid to take readings. They come as portable instruments or built-in systems.
The sensors use their electrodes to detect voltage differences. This gives fast and cheap results for many uses. A higher ORP value usually means the water is cleaner and better disinfected.
ORP probes are easy to use and safe. They are often used with other tools that measure water quality. These tools include pH sensors, dissolved oxygen sensors, electrical conductivity (EC) sensors, and chlorine sensors.
If you are not sure which sensor to choose, please ask for help. We can help you find the best water monitoring solution for your needs.
How does an ORP sensor work?
ORP sensors have two main parts. One part is a measuring electrode. It is often made of precious metals like platinum or gold. The other part is a reference electrode, which is often Ag/AgCl.
When the sensor is put in the solution, the measuring electrode works with both oxidized and reduced substances. This creates a difference in voltage compared to the reference electrode. This voltage difference provides the ORP measurement.
It’s important to know that ORP sensors measure the total effect of all redox reactions in the solution. They measure reactions that involve more than one specific substance. As such, the ORP value represents the overall redox condition of the solution.
Factors influencing ORP
Many factors work together to affect a solution’s Oxidation-Reduction Potential. These drivers can be grouped into three types: chemical factors, physical factors, and biological factors.
Chemical factors include pH levels, dissolved oxygen, and the presence of agents that can oxidize or reduce. Physical factors include temperature and exposure to UV light.
Biological factors relate to the types and levels of microbial activity. They either take part in redox reactions or change the environmental conditions that influence those reactions.
1. **pH**
One main factor that affects the Oxidation-Reduction Potential (ORP) in water is the change in pH levels. This connection exists because hydrogen ions (H⁺) play a key role in redox reactions.
Changes in pH directly affect the energy that drives electron transfer processes. When pH goes up, the ORP value goes down. When pH goes down, the ORP value goes up.
For example, when chlorine is used to clean swimming pools, it dissolves. This creates a balance between hypochlorous acid (HOCl) and hypochlorite ions (OCl⁻).
HOCl is a strong oxidant and disinfectant. In contrast, OCl⁻ is much weaker. Its ability to kill bacteria is only 1/80 to 1/300 that of HOCl. The pH level of the water is important for this balance.
**Effect of pH on Available Chlorine Form and ORP**
At a pH range of 7.0 to 7.5, most available chlorine is in the stronger form of HOCl. This boosts the water’s ability to oxidize and disinfect while keeping a high ORP value.
When the pH goes above 8.0, HOCl breaks down into the less effective OCl⁻. Even if the total chlorine level stays the same, the water’s ability to disinfect goes down a lot. This is shown by a big drop in the ORP value.
2. **Dissolved Oxygen**
Oxygen is a strong oxidant. It has a big impact on the ORP value when it dissolves in water. Higher levels of dissolved oxygen help microbes break down organic matter. This happens because oxygen allows electrons to move from organic compounds.
The oxidation-reduction potential (ORP) goes up as a result. For example, according to an EPA water quality report, raising dissolved oxygen (DO) from 2 mg/L to 8 mg/L enhances ORP from approximately 300 mV to 500 mV.
Aeration can greatly increase the levels of dissolved oxygen. This increase boosts the ORP value and helps aerobic microorganisms. These tiny organisms help break down organic matter and support nitrification.
If organic matter and reducing agents use up more dissolved oxygen than it can be replaced, DO levels fall. This causes a lower ORP and changes the water from aerobic to anoxic or anaerobic states.
3. **Oxidants and Reductants**
Oxidants are substances that can take in electrons. As their concentration goes up, water pulls in electrons more strongly. This raises the Oxidation-Reduction Potential value. Typical oxidizing agents present in water are hypochlorous acid, ozone, chlorine, and permanganate ions.
More of these substances result in higher ORP values. This shows a strong oxidizing environment. For example, swimming pool water with an ORP over +650 mV shows good sterilization and disinfection.
Reductants give off electrons into the water. This fights oxidants and lowers the ORP value by making more electrons available. Common reducing agents found in water are organic compounds, sulfide ions, nitrite, ferrous ions, and hydrogen gas.
High levels of reductants lower ORP values a lot. This makes a reducing environment. You can often find this in sewage systems or polluted water.
4. **Temperature**
Temperature plays a key role in speeding up chemical and biological reactions in water. It affects how fast oxidants and reductants are produced and used.
This, in turn, changes the ORP values. Higher temperatures reduce the amount of oxygen that can dissolve in water. This leads to more changes in ORP.
The link between temperature and ORP is clear. Calibration standards for ORP values depend on temperature. The Zobell standard solution has an ORP value of 228 mV at 25°C.
It increases to 241 mV at 15°C. It is important to consider these differences when choosing ORP sensors. Selecting models with temperature compensation ensures accurate measurements in changing conditions.
5. Ultraviolet light
Ultraviolet (UV) light from the sun is a strong energy source. It can break down some oxidants in water. These include hypochlorous acid (HOCl) and ozone (O₃). This happens through a process called photolysis.
This process reduces the amount of oxidants. This leads to a lower Oxidation-Reduction Potential (ORP).
UV light has a strong effect on outdoor pools and open water. It causes a daily change in ORP levels. ORP values often reach their lowest point in the afternoon.
This occurs when the sunlight is at its strongest. They then rise slowly overnight.
6. Microorganisms
Microbial activity plays a key role in changing ORP levels in various water environments. Microorganisms use their metabolic processes to perform redox reactions. This affects the electron levels in the water. As a result, it changes the Oxidation-Reduction Potential.
Beyond metabolism, the specific types of microorganisms present also impact ORP dynamics. Different bacterial communities have their own enzyme systems, electron transport chains, and substrate preferences. This leads to different contributions to ORP, even in similar environmental conditions.
Aerobic nitrifying bacteria, such as Nitrosomonas, thrive in places with plenty of oxygen. They like ORP levels between +300 and +500 mV. In these conditions, they change ammonia into nitrogen compounds.
Denitrifying bacteria, such as Pseudomonas, like to live in low-oxygen environments. They work best when ORP levels are between +50 and +250 mV. In these conditions, they turn nitrates into nitrogen gas.
Iron-reducing bacteria grow well in places with low to moderate oxidation-reduction potential (ORP). This range is between -50 and +100 mV. They use Fe³⁺ as an electron acceptor.
Sulfate-reducing bacteria and methanogens work in very low oxygen conditions. They thrive in ORP ranges of about -100 mV to -200 mV. These bacteria produce hydrogen sulfide and methane. Changes in ORP values can show what types of microbes are in the water and what they are doing.