what is evapo and water evaporation?
Water has three forms: liquid, gas, and solid. These forms can change based on the environment. At normal temperatures, water slowly turns into vapor and spreads into the air. This process is known as evaporation.
What is evapo ? This can happen at any temperature. For example, water in a bucket can dry up when it is in the sun.
Puddles on the ground slowly vanish. Wet clothes also dry out. These are all common examples of evaporation.
Why Can Evaporation Occur at Any Temperature?
Evaporation occurs when energetic molecules in a liquid break free from their bonds. They then change into water vapor. In a liquid water, molecules move fast and in random ways. Their movement does not depend on the temperature.
Temperature shows the average energy of all molecules. Some molecules need more energy than this average. This extra energy helps them break through surface tension and turn into vapor. Even when it is cold, these high-energy molecules can still have enough energy to escape into the air.
The Boltzmann distribution shows that fewer molecules turn into gas at lower temperatures. Evaporation happens when some molecules break free.
At higher temperatures, more molecules get the energy they need to evaporate. This makes evaporation happen faster. In contrast, the rate of evaporation slows down a lot at lower temperatures.
Evaporation in Nature
Several key factors influence evaporation: **temperature**, **humidity**, **wind speed**, and **air pressure**.
How Does Temperature Affect Evaporation?
The hot water it is, the quicker water evaporates. Heat makes the molecules in the liquid move faster. This raises their kinetic energy. As a result, more molecules can break free from the surface and turn into water vapor.
For example, think of a pot of water heating on a stove. As the temperature goes up, more steam escapes into the air.
This steam shows that water is evaporating faster. Higher temperatures make molecules move more. This speeds up evaporation and transpiration.
How Does Humidity Affect Evaporation?
High humidity means the air has a lot of water vapor. It is close to being full. In these conditions, evaporation slows down. This happens because the air can hold only so much moisture.
High humidity adds more water vapor to the air. This makes it harder for water to turn into vapor. When the air has a lot of water vapor, evaporation does not work as well.
How Does Wind Speed Affect Evaporation?
Air pressure serves as an indicator of how dense or “thick” the atmosphere is. High air pressure creates a denser atmosphere. This slows down how fast water molecules can move from the liquid surface into the air.
This naturally lowers the rate of evaporation. When air pressure is low, the atmosphere is less thick. This makes it easier for water molecules to leave the surface.
Think of it like people getting on a bus. When the bus is almost empty, it is easy to find a seat. But when the bus is almost full, it is much harder for new passengers to get on.
Similarly, low atmospheric pressure creates more space for water molecules to escape into the air. This leads to faster evaporation.
Evaporation vs. Volatilization
**Key differences in properties:**
Evaporation is when molecules at the surface of a liquid escape and turn into vapor. This happens because of the random movement of water molecules in the liquid state.
Volatilization means the movement and release of molecules into the air from a substance. This substance can be a liquid or a solid. Unlike evaporation, volatility is not dependent on temperature.
**Occurrence conditions:**
Evaporation can occur at any temperature. The rate of evaporation increases as the temperature goes up. On the other hand, volatilization mainly involves some substances, like organic compounds. It is mostly not changed by temperature.
**Characteristics and influencing factors:**
Process of evaporation that happens at the surface of a liquid. Only the molecules at the top turn into vapor. This process usually occurs slowly and takes in heat.
The liquid and the area around it cool down. Things like surface area, temperature, and air movement affect how fast evaporation occurs.
Volatilization is a process where some liquids, like ether or alcohol, spread into the air at room temperature. Although it doesn’t require heat to occur, the process often takes longer than evaporation.
How Does Evaporation Affect Weather?
Evaporation has a big impact on the weather. It affects how energy moves and the water cycle.
**Impact on Energy Exchange:**
When water turns from liquid to vapor, it takes in a lot of heat. This is called latent heat energy. It is about 2260 KJ per kilogram. The energy needed for this change is very high.
To evaporate 1 kg of water, you need the same energy as heating 538 kg of water by 1°C. This process takes heat from the area around it. As a result, it creates a evaporative cooling effect in the nearby environment.
**Impact on the Water Cycle:**
Evaporation plays a key role in the Earth’s water cycle. Water from mountains flows down to plains and then into oceans due to gravity.
Evaporation changes water from oceans into water vapor. Oceans cover more than 70% of the Earth. This process happens against gravity.
The wind brings this vapor to land and mountains. There, it turns into clouds and eventually falls as rain or snow. The cycle affects weather patterns and helps control the climate.
Measuring Outdoor Evaporation
1. Manual Calculation:
Using an evaporating dish is a common way to measure how fast water evaporates. This method uses a big container. It is filled with a certain amount of water. You place it in the area you want to study.
You then track the changes in water level over time with a measuring device or markers. We will now calculate the evaporation rate using this data.
Key considerations:
The container should have a smooth surface. It must resist rust and have a uniform thickness. This helps reduce changes in surface tension.
You need to manage outside factors like rain or extra moisture to get accurate measurements.
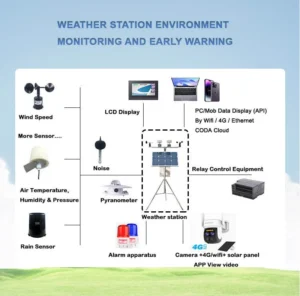
2. Evaporation Sensor:
An evaporation sensor is often used in outdoor weather stations because it is accurate and automated. These devices measure evaporation by tracking weight changes in a liquid container.
They work by using changes in pressure. They can also connect to sensors that check for rain or snow. This helps with automated climate monitoring.
3. Reverse Osmosis Membrane
The reverse osmosis membrane method works based on osmotic pressure. In this method, a clear film is placed on the water’s surface. This film allows water molecules to pass through but blocks salt and other impurities.
As water molecules move through the membrane, osmotic pressure builds up. You can find the evaporation rate by measuring changes in the weight and pressure of the membrane. The main feature of this membrane is that it can select what goes through. It allows water molecules to pass through easily but keeps out contaminants.
4. Turbine Flowmeter
The turbine flowmeter method uses a spinning sensor to measure how fast water flows. Researchers then change this rate into evaporation data.
This technique is more complex than reverse osmosis. It needs several tools to work together. Accurate calibration and fine-tuning are key for reliable measurements. This method is hard, but it is very accurate.
Conclusion
Nature is both beautiful and complex. It reveals the secrets of life’s cycles. Evaporation plays an important role in this balance.
This process often goes unnoticed, but it has a big impact on our environment. Without evaporation, water vapor cannot become rain. Rivers would become still, and ecosystems that rely on the water cycle would be harmed.
Evaporation is a key link between the Earth’s surface and the atmosphere. It helps rivers flow and shapes ecosystems. Its effects reach beyond nature. It also impacts key human activities, such as farming and large water management projects.
This includes projects that divert water and the South-to-North Water Transfer Project. Managing evaporation is key for sustainable development and human survival.
Studying evaporation gives us important ideas and practical uses. These ideas help the environment. They assist with reforestation and help plan how to use land.